
The origin of van der Waals dispersion forcesĪttractions are electrical in nature. Helium's intermolecular attractions are even weaker - the molecules won't stick together to form a liquid until the temperature drops to 4 K (-269☌).ĭispersion forces (one of the two types of van der Waals force we are dealing with on this page) are also known as "London forces" (named after Fritz London who first suggested how they might arise).
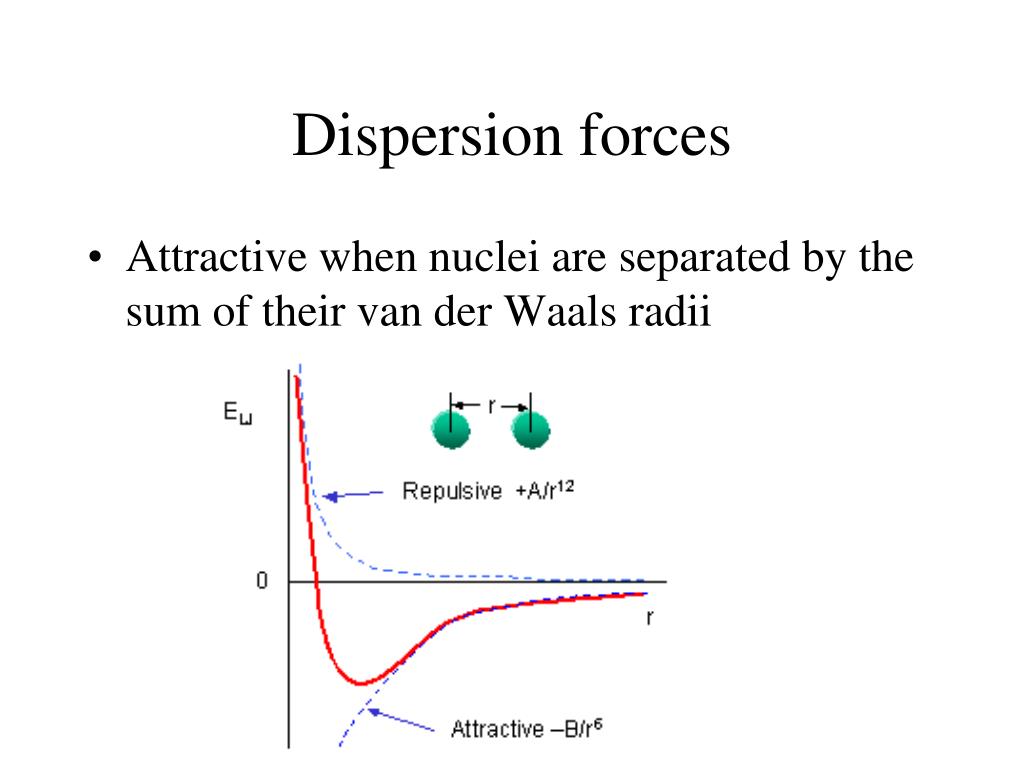
In hydrogen's case the attractions are so weak that the molecules have to be cooled to 21 K (-252☌) before the attractions are enough to condense the hydrogen as a liquid. Even in a gas like hydrogen, H 2, if you slow the molecules down by cooling the gas, the attractions are large enough for the molecules to stick together eventually to form a liquid and then a solid.

The term "intramolecular" won't be used again on this site.Īll molecules experience intermolecular attractions, although in some cases those attractions are very weak. These two words are so confusingly similar that it is safer to abandon one of them and never use it. The forces of attraction which hold an individual molecule together (for example, the covalent bonds) are known as intramolecular attractions. Intermolecular attractions are attractions between one molecule and a neighbouring molecule. Intermolecular versus intramolecular bonds If you are also interested in hydrogen bonding there is a link at the bottom of the page. This page explains the origin of the two weaker forms of intermolecular attractions - van der Waals dispersion forces and dipole-dipole attractions. INTERMOLECULAR BONDING - VAN DER WAALS FORCES

Intermolecular bonding - van der Waals forces


 0 kommentar(er)
0 kommentar(er)
